RetaLac®
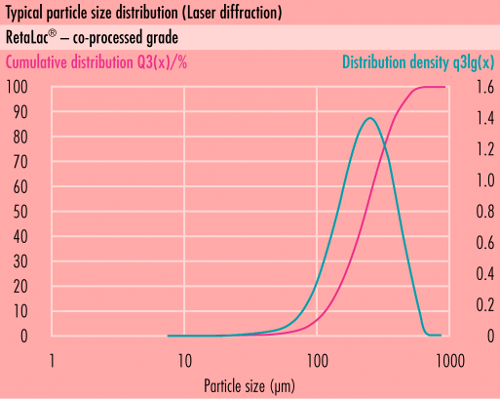
MEGGLEs co-processed excipient, RetaLac®, appears as a white, or almost white odorless powder, which is freely flowing and partially soluble in cold water. It comprises equal parts of hypromellose (type 2208, a.k.a. “K-type”) with a nominal viscosity of 4000 mPa·s, together with milled ?lpha-lactose monohydrate grade, both of compendial quality. A specialized spray-agglomeration process generates textured, highly structured particles with d50 in the range of many directly-compressible excipients, 100 µm to 200 µm, typically 125 µm.
Lactose identification is performed according to Ph. Eur. lactose monohydrate identification test method C: a red coloration appears. Verification of hypromellose is performed according to the hypromellose monograph, identification test method B, E also Ph. Eur.: a gelation of solution is detectable at >50°C.
Shelf life / Retest:
24 months
Standard Packaging:
12 kg - Plastic Drum with PE-EVOH-PE Inliner
Particle size distribution
[Mechanical sieve shaker]
- <63 µm: NMT 25%
- <250 µm: NLT 80%
Typical Values
- Bulk Density [g/l]: 340
- Tapped Density [g/l] : 460
- Hausner factor: 1.35
- Carrs index: 26.09 %
Benefits
- Direct compression of modified release formulations
- Superior processibility compared to corresponding wet granulated and physical admixture of parent ingredients
- Meets compendial requirements
- Drug release kinetics are predominantly controlled by pure diffusion
- Unaffected by acidic conditions (between pH 1 and 7)
- Dissolution can be quit
Areas of Application
- Tabletting - Direct Compression, also for multi unit and mini tablets
- Tabletting - Roller Compaction
- Preparation of aqueous HPMC-formulations
- Spheronization, Extrusion