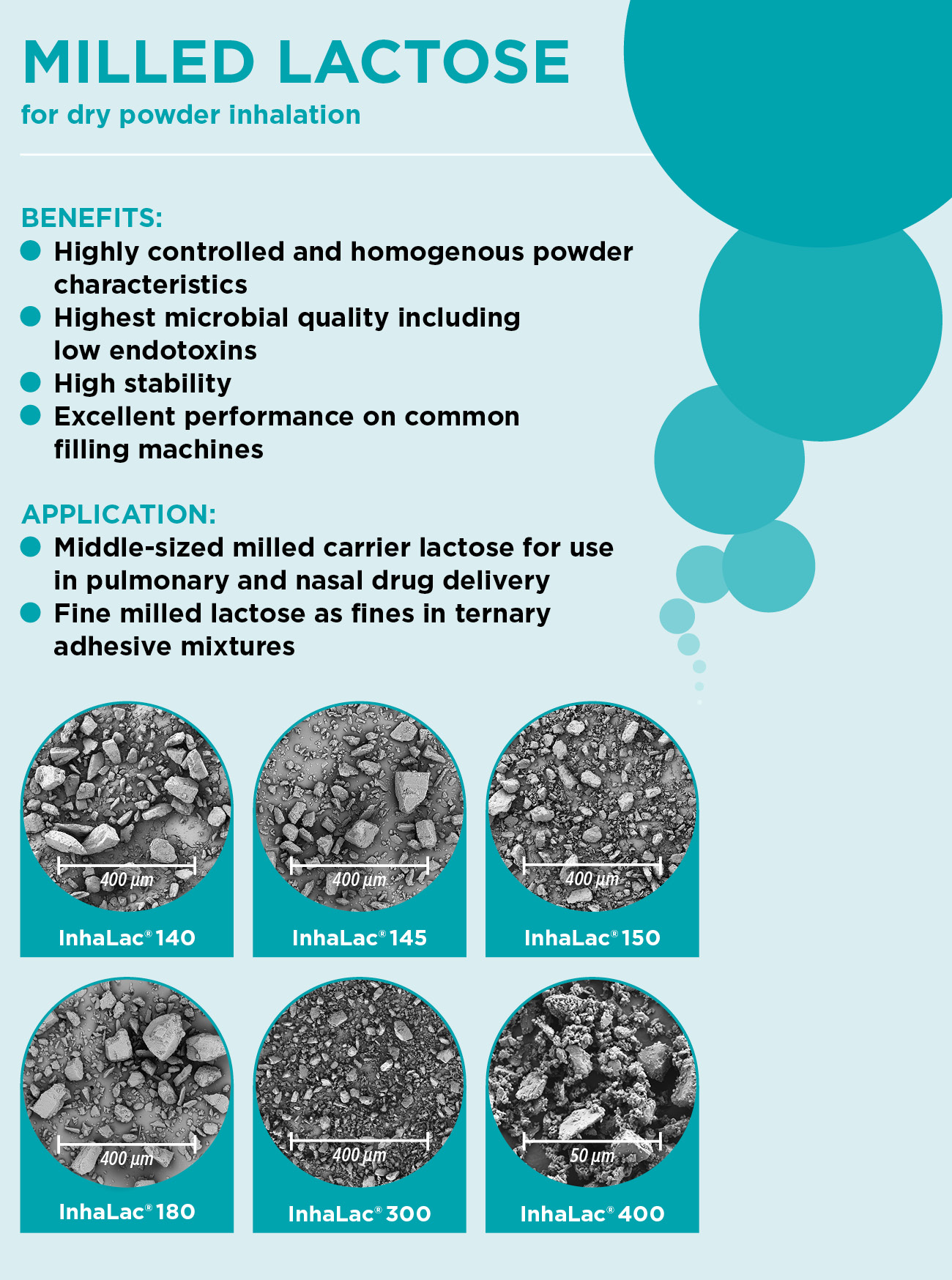
Milled lactose for pulmonary and nasal drug delivery
MEGGLE has it.
The right milled lactose for dry powder inhalation
The delivery of active pharmaceutical ingredients (APIs) via the lung is becoming increasingly important as more and more patients all over the world suffer from chronic respiratory diseases.
NEW: InhaLac® 145
MEGGLE´s extension of the InhaLac® product family. InhaLac® 145 is a new product within the family of milled inhalative lactose. It consists of irregularly shaped particles with a strict controlled and specified particle size. InhaLac® 145 has a typical rough surface, cohesiveness and flowability of milled lactoses.
InhaLac® 145 is a middle sized carrier lactose with mean particle size of 35 µm and approximately 11% of particles below 5 µm. The expansion of MEGGLE´s InhaLac® product family allows the formulator to choose the optimal DPI lactose grade for each application and finetune each formulation for superior performance. Click for more information.
InhaLac® 180
InhaLac® 180 is tailored to dry powder inhalation (DPI) applications with a highly uniform particle size distribution (PSD) of X10=5-15µm, X50=50-100µm, and X90=120-160µm and characterized by the typical flow and surface characteristics of milled lactose. With the launch, the quality of MEGGLE’s lactose excipient meets a specific customer need while expanding the toolbox for developers.
InhaLac® 300
MEGGLE´s extension of the InhaLac® product family – closing the gap between medium sized carrier lactose and fine milled grades for dry powder inhalation.
InhaLac® 300 – a new inhalative lactose grade with specific particle size distribution is characterized by the typical flow- and surface-characteristics of fine milled lactose grades. This provides an additional tool for the formulator to tune and optimize the performance of the DPI product. InhaLac® 300 is a lactose grade with 90% of the particles below 35-50 µm (Sympatec) or 40-56 µm (Malvern).
InhaLac® 140 and InhaLac® 150: Milled carrier lactose
Dry Powder Inhaler (DPI) are enjoying still an increasing popularity. This is due to their advantages, such as ease of use, small size, portability, and the lack of requirement of breath actuation coordination. Because they are propellant-free, they are environmentally friendly. Furthermore, as solid-particle formulations they are comparatively stable. Usually, this dosage form contains a device, one or more APIs and an excipient that improves the powder properties of the formulation. Features such as particle size are fundamental factors in the design of DPIs.
Most DPI formulations employ a carrier excipient to provide bulk and flow. The most common carrier used is lactose which can make up to 99 % of the formulation. It has been shown that not only the particle size distribution of the carrier highly influences the performance of the formulation but also surface properties and flow characteristics can be used to modify drug delivery. Therefore, MEGGLE provides with InhaLac® 140 and InhaLac® 150 two mid-sized milled carrier lactose grades suitable for different device types exhibiting powder characteristics typical for milled lactose grades with known high quality standards for inhalation.
Information / Sample request:
InhaLac® 400: Fine tune your DPI formulation
Besides the carrier lactose and the active pharmaceutical ingredient, many dry powder inhalation formulations contain additionally fine milled or micronized lactose. The addition of lactose fines (< 15 µm) in ternary powder mixtures for inhalation is well-known to increase the fine particle fraction (FPF, < 5 µm).
Numerous scientific publications have shown that the addition of fine milled lactose to coarser lactose, which acts as carrier for the API in DPIs, can drastically improve the DPI’s performance by increasing its fine particle fraction. One mechanism, which is discussed for the performance improvement is called high energy site theory, where the fine milled lactose covers the so-called high energy sites of the coarser lactose, preventing the API from strong attachment to its carrier.
MEGGLE’s InhaLac® 400 is a finely milled alpha-lactose monohydrate with a typical median particle size of x50 = 8 μm.
Batch-to-batch consistency for all lactose products is due to MEGGLE’s technical expertise in lactose manufacture. Our stringent release criteria and constant process control ensure our products’ consistency and quality.
MEGGLE’s InhaLac® grades comply with the current harmonized USP-NF, Ph. Eur. and JP monographs. In order to meet the special requirements for pulmonary drug delivery, additional and in some cases even stricter specification limits are in place for all InhaLac® grades. These exceed even those currently required by the pharmacopoeias.
MEGGLE´s milled lactose grade for DPI is available under the trade name
InhaLac® 140, InhaLac® 145, InhaLac® 150, InhaLac® 180, InhaLac® 300 and InhaLac® 400
MEGGLE has it.
The right milled lactose for dry powder inhalation
The delivery of active pharmaceutical ingredients (APIs) via the lung is becoming increasingly important as more and more patients all over the world suffer from chronic respiratory diseases.

NEW: InhaLac® 145
MEGGLE´s extension of the InhaLac® product family. InhaLac® 145 is a new product within the family of milled inhalative lactose. It consists of irregularly shaped particles with a strict controlled and specified particle size. InhaLac® 145 has a typical rough surface, cohesiveness and flowability of milled lactoses.
InhaLac® 145 is a middle sized carrier lactose with mean particle size of 35 µm and approximately 11% of particles below 5 µm. The expansion of MEGGLE´s InhaLac® product family allows the formulator to choose the optimal DPI lactose grade for each application and finetune each formulation for superior performance. Click for more information.
InhaLac® 180
InhaLac® 180 is tailored to dry powder inhalation (DPI) applications with a highly uniform particle size distribution (PSD) of X10=5-15µm, X50=50-100µm, and X90=120-160µm and characterized by the typical flow and surface characteristics of milled lactose. With the launch, the quality of MEGGLE’s lactose excipient meets a specific customer need while expanding the toolbox for developers.
InhaLac® 300
MEGGLE´s extension of the InhaLac® product family – closing the gap between medium sized carrier lactose and fine milled grades for dry powder inhalation.
InhaLac® 300 – a new inhalative lactose grade with specific particle size distribution is characterized by the typical flow- and surface-characteristics of fine milled lactose grades. This provides an additional tool for the formulator to tune and optimize the performance of the DPI product. InhaLac® 300 is a lactose grade with 90% of the particles below 35-50 µm (Sympatec) or 40-56 µm (Malvern).
InhaLac® 140 and InhaLac® 150: Milled carrier lactose
Dry Powder Inhaler (DPI) are enjoying still an increasing popularity. This is due to their advantages, such as ease of use, small size, portability, and the lack of requirement of breath actuation coordination. Because they are propellant-free, they are environmentally friendly. Furthermore, as solid-particle formulations they are comparatively stable. Usually, this dosage form contains a device, one or more APIs and an excipient that improves the powder properties of the formulation. Features such as particle size are fundamental factors in the design of DPIs.
Most DPI formulations employ a carrier excipient to provide bulk and flow. The most common carrier used is lactose which can make up to 99 % of the formulation. It has been shown that not only the particle size distribution of the carrier highly influences the performance of the formulation but also surface properties and flow characteristics can be used to modify drug delivery. Therefore, MEGGLE provides with InhaLac® 140 and InhaLac® 150 two mid-sized milled carrier lactose grades suitable for different device types exhibiting powder characteristics typical for milled lactose grades with known high quality standards for inhalation.
InhaLac® 400: Fine tune your DPI formulation
Besides the carrier lactose and the active pharmaceutical ingredient, many dry powder inhalation formulations contain additionally fine milled or micronized lactose.
The addition of lactose fines (< 15 µm) in ternary powder mixtures for inhalation is well-known to increase the fine particle fraction (FPF, < 5 µm).
Numerous scientific publications have shown that the addition of fine milled lactose to coarser lactose, which acts as carrier for the API in DPIs, can drastically improve the DPI’s performance by increasing its fine particle fraction. One mechanism, which is discussed for the performance improvement is called high energy site theory, where the fine milled lactose covers the so-called high energy sites of the coarser lactose, preventing the API from strong attachment to its carrier.
MEGGLE’s InhaLac® 400 is a finely milled alpha-lactose monohydrate with a typical median particle size of x50 = 8 μm.
Batch-to-batch consistency for all lactose products is due to MEGGLE’s technical expertise in lactose manufacture. Our stringent release criteria and constant process control ensure our products’ consistency and quality.
MEGGLE’s InhaLac® grades comply with the current harmonized USP-NF, Ph. Eur. and JP monographs. In order to meet the special requirements for pulmonary drug delivery, additional and in some cases even stricter specification limits are in place for all InhaLac® grades. These exceed even those currently required by the pharmacopoeias.
MEGGLE´s milled lactose grade for DPI is available under the trade name
InhaLac® 140, InhaLac® 145, InhaLac® 150, InhaLac® 180, InhaLac® 300 and InhaLac® 400
